Buy (4ANPP) Depropionylfentanyl CAS 21409-26-7
Buy (4ANPP) Depropionylfentanyl CAS 21409-26-7
Description:
Synonyms:
- 1-(2-Phenylethyl)-4-phenylaminopiperidine
- 1-Phenethyl-N-phenylpiperidin-4-amine
- 4-Anilino-1-(2-phenethyl)piperidine
- 4-Anilino-1-(β-phenethyl)piperidine
- 4-Anilino-1-phenethylpiperidine
- 4-Piperidinamine, N-phenyl-1-(2-phenylethyl)-
- 4-anilino-N-fenetilpiperidina (ANPP)
- 4Anpp
- ANPP, N-phenyl-1-(2-phenylethyl)piperidin-4-amine
- Depropionylfentanyl
4-ANPP, also known as 4-anilino-N-phenethylpiperidine (4-ANPP), 4-aminophenyl-1-phenethylpiperidine, or despropionyl fentanyl,[3] is a direct precursor to fentanyl and acetylfentanyl. It is commonly found as a contaminant in samples of drugs containing fentanyl, which may include samples represented by the supplier as heroin or other opioids.[4] It is not psychoactive and is present only as a result of improper chemical purification.
4-ANPP is useful in the synthesis of pharmaceuticals, primarily fentanyl and related analogs. Paul Janssen (founder of Janssen Pharmaceutica) first synthesized fentanyl in 1960 using a similar method, with Benzylfentanyl as an intermediate.[5] The following synthesis, developed by an individual under the pseudonym of Siegfried, involves the reductive amination of N–phenethyl-4-piperidinone (NPP) with aniline to make to 4-ANPP. This product is reacted with propionyl chloride or acetyl chloride to form either fentanyl or acetylfentanyl.
See also
1-phenethyl-N-phenylpiperidin-4-amine
Description
Properties
IUPAC Name |
N-phenyl-1-(2-phenylethyl)piperidin-4-amine | |
|---|---|---|
InChI |
InChI=1S/C19H24N2/c1-3-7-17(8-4-1)11-14-21-15-12-19(13-16-21)20-18-9-5-2-6-10-18/h1-10,19-20H,11-16H2 | |
InChI Key |
ZCMDXDQUYIWEKB-UHFFFAOYSA-N | |
Canonical SMILES |
C1CN(CCC1NC2=CC=CC=C2)CCC3=CC=CC=C3 | |
Molecular Formula |
C19H24N2 | |
DSSTOX Substance ID |
DTXSID40175680 | |
Molecular Weight |
280.4 g/mol | |
CAS No. |
21409-26-7 |
Synthetic Methodologies and Chemical Transformations of 1 Phenethyl N Phenylpiperidin 4 Amine
Established Synthetic Routes to 1-Phenethyl-N-phenylpiperidin-4-amine
The synthesis of this compound, also known as 4-anilino-N-phenethylpiperidine (ANPP), can be achieved through several established chemical pathways. These routes primarily involve the formation of the core piperidine (B6355638) scaffold and the subsequent introduction of the N-phenethyl and N-phenylamino substituents.
Reductive amination stands out as a prevalent and efficient method for the synthesis of this compound. This approach typically commences with the reaction of N-phenethyl-4-piperidone (NPP) and aniline (B41778). The initial reaction forms an imine or enamine intermediate, which is then reduced in situ to the desired secondary amine.
A common protocol involves the use of sodium triacetoxyborohydride as the reducing agent. This reagent is favored for its mildness and selectivity, allowing for the efficient conversion of the imine to the amine without reducing other functional groups. The reaction is typically carried out in a suitable solvent, such as dichloromethane or dichloroethane, often in the presence of an acid catalyst like acetic acid to facilitate imine formation. Alternative reducing agents, such as sodium borohydride, have also been employed. Another variation of this method involves a one-pot reaction where 4-piperidone is first reductively alkylated with phenylacetaldehyde to form N-phenethyl-4-piperidone, which then undergoes reductive amination with aniline in the same reaction vessel.
| Reactants | Reducing Agent | Solvent | Yield (%) |
| N-phenethyl-4-piperidone, Aniline | Sodium triacetoxyborohydride | Dichloromethane, Acetic Acid | 91 |
| N-phenethyl-4-piperidone, Aniline | Sodium borohydride | Not specified | Not specified |
| 4-Piperidone, Phenylacetaldehyde, Aniline | Sodium triacetoxyborohydride | Not specified | Not specified |
This table summarizes common reductive amination protocols for the synthesis of this compound.
An alternative synthetic strategy involves the direct alkylation of N-phenylpiperidin-4-amine with a phenethyl halide, such as 2-(bromoethyl)benzene or 2-phenylethyl chloride. This reaction is typically carried out in the presence of a base to neutralize the hydrogen halide formed during the reaction. The choice of base and solvent can influence the reaction’s efficiency and yield. This method is contingent on the availability of the N-phenylpiperidin-4-amine precursor, which itself can be synthesized from 4-piperidone.
Beyond the direct functionalization of pre-existing piperidine rings, alternative methods focus on the initial construction of the core piperidine scaffold. One notable approach is the Dieckmann condensation. This intramolecular cyclization of a diester, formed from the reaction of phenethylamine with two equivalents of an acrylate ester (like methyl acrylate), yields a β-ketoester. Subsequent hydrolysis and decarboxylation of this cyclic product afford N-phenethyl-4-piperidone, a key intermediate that can then be converted to this compound via reductive amination as previously described.
Another approach starts from pyridine derivatives. For instance, 4-anilinopyridine can be acylated and then alkylated with a phenethyl halide to form a pyridinium salt. Subsequent reduction of the pyridine ring yields the desired piperidine structure.
Optimization of Reaction Conditions and Yields
Significant research has been dedicated to optimizing the reaction conditions for the synthesis of this compound to maximize yields and purity. For the widely used reductive amination of N-phenethyl-4-piperidone with aniline, studies have shown that using sodium triacetoxyborohydride in the presence of acetic acid can lead to excellent yields, often exceeding 90%. The use of cesium carbonate as the base in the alkylation of 4-piperidone with 2-(bromoethyl)benzene has also been reported to provide high yields of the intermediate N-phenethyl-4-piperidone, in the range of 88%.
| Synthetic Step | Reagents and Conditions | Yield (%) |
| Alkylation of 4-piperidone | 2-(bromoethyl)benzene, Cesium carbonate | 88 |
| Reductive Amination of NPP | Aniline, Sodium triacetoxyborohydride, Acetic acid | 91 |
| Acylation to Fentanyl | Propionyl chloride, Diisopropylethylamine | 95 |
This table highlights optimized yields for key steps in a common synthetic route to fentanyl, starting from 4-piperidone.
-
-

N-Phenyl-1-(2-phenylethyl)piperidin-4-amine
Controlled ProductCAS:
Formula:C19H24N2Color and Shape:NeatMolecular weight:280.41 -
4-Aminophenyl-1-phenethylpiperidine
Controlled ProductCAS:
Impurity Fentanyl Impurity D / Fentanyl USP Related Compound E
Applications 4-Aminophenyl-1-phenethylpiperidine is a metabolite of 3H-Fentanyl (F274990), which is used as an analgesic.Controlled Substance.
References Labroo, R., et al.: Drug Metab., Disposition, 25, 1072 (1997); Zernig, G., et al.: Life Sci., 57, 2113 (1995); Bot, G., et al.: J. Pharmacol. Exper. Therap., 285, 1207 (1998);Formula:C19H24N2Color and Shape:NeatMolecular weight:280.41 -

4-ANPP
Controlled ProductCAS:
4-ANPP is a solution in acetonitrile that is commonly used in the production of biodiesel. It is also used as an intermediate for the synthesis of various compounds, including β-ionone and hydroxyapatite. 4-ANPP has been shown to have controlled release properties, making it suitable for use in controlled-release products. It can be used as a phosphatase inhibitor, preventing the breakdown of phosphates in biological systems. Additionally, 4-ANPP has been found to enhance biomass production and improve cell growth in certain applications. Its unique properties make it a versatile compound for various industries, including pharmaceuticals, agriculture, and research.
Formula:C19H24N2Purity:Min. 95%Color and Shape:PowderMolecular weight:280.41 g/molRef: 3D-IP17824
1mg179.00€
Estimated delivery time: ~24 days -
4-Aminophenyl-1-phenethylpiperidine (Fentanyl Impurity) (1mg/ml in Acetonitrile)
Controlled ProductCAS:
Formula:C19H24N2Color and Shape:ColourlessMolecular weight:280.41Ref: TR-KIT2895
1x1ml1,013.00€
Estimated delivery time: ~54 days -

Fentanyl Related Compound E CII (N-Phenyl-1-(2-phenylethyl)-4-piperidinamine)
Controlled ProductCAS:
4-anilino-n-phenethylpiperidine (anpp)Formula:C19H24N2Color and Shape:White Light Brown Crystalline PowderMolecular weight:280.19395Order 4-Anilino-N-phenethylpiperidine (4-ANPP) Precursor
Order 4-Anilino-N-phenethylpiperidine (4-ANPP) Precursor
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP,
4-ANPP, also known as 4-anilino-N-phenethylpiperidine (4-ANPP), 4-aminophenyl-1-phenethylpiperidine, or despropionyl fentanyl,[3] is a direct precursor to fentanyl and acetylfentanyl. It is commonly found as a contaminant in samples of drugs containing fentanyl, which may include samples represented by the supplier as heroin or other opioids.[4] It is not psychoactive and is present only as a result of improper chemical purification. Order 4-Anilino-N-phenethylpiperidine (4-ANPP) Precursor
4-ANPP is useful in the synthesis of pharmaceuticals, primarily fentanyl and related analogs. Paul Janssen (founder of Janssen Pharmaceutica) first synthesized fentanyl in 1960 using a similar method, with Benzylfentanyl as an intermediate.[5] The following synthesis, developed by an individual under the pseudonym of Siegfried, involves the reductive amination of N–phenethyl-4-piperidinone (NPP) with aniline to make to 4-ANPP. This product is reacted with propionyl chloride or acetyl chloride to form either fentanyl or acetylfentanyl. Order 4-Anilino-N-phenethylpiperidine (4-ANPP) Precursor
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP). 4-ANPP-13C6 (CRM) is a certified reference material intended for use as an internal standard for the quantification of 4-ANPP by GC- or LC-MS. 4-ANPP is categorized as a piperidinamine. It is an intermediate in the synthesis of fentanyl from N-phenethyl-4-piperidone .1 As such, 4-ANPP has been used as a precursor for the manufacture of fentanyl and related opioids. 4-ANPP is also an impurity found in fentanyl preparations. It is a known metabolite of acetyl fentanyl , butyryl fentanyl , furanyl fentanyl , acrylfentanyl , and fentanyl.2,3,4,5 4-ANPP-13C6 is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications. Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
4-ANPP, also known as 4-anilino-N-phenethylpiperidine (4-ANPP), 4-aminophenyl-1-phenethylpiperidine, or despropionyl fentanyl,[3] is a direct precursor to fentanyl and acetylfentanyl. It is commonly found as a contaminant in samples of drugs containing fentanyl, which may include samples represented by the supplier as heroin or other opioids.[4] It is not psychoactive and is present only as a result of improper chemical purification. Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
4-ANPP is useful in the synthesis of pharmaceuticals, primarily fentanyl and related analogs. Paul Janssen (founder of Janssen Pharmaceutica) first synthesized fentanyl in 1960 using a similar method, with Benzylfentanyl as an intermediate.[5] The following synthesis, developed by an individual under the pseudonym of Siegfried, involves the reductive amination of N–phenethyl-4-piperidinone (NPP) with aniline to make to 4-ANPP. This product is reacted with propionyl chloride or acetyl chloride to form either fentanyl or acetylfentanyl. Order 4-Anilino-N-phenethylpiperidine (4-ANPP) Precursor
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
Buy desproprionyl fentanyl, 4-anilino-N-phenethylpiperidine, 4-ANPP, ANPP
- 4-Aminophenyl-1-phenethylpiperidine-13C6
- 4-Anilino-N-phenethylpiperidine-13C6
- Despropionyl fentanyl-13C6
Technical Information
Formal Name: 1-phenethyl-N-(phenyl-13C6) piperidin-4- amine CAS Number 2748319-07-3Molecular FormulaC13[13C]6H24N2Formula Weight286.4Purity≥98%Formulation(Request formulation change)A neat solidSMILES[13C]1(NC2CCN(CCC3=CC=CC=C3)CC2)=[13CH][13CH]=[13CH][13CH]=[13CH]1InChi CodeInChI=1S/C19H24N2/c1-3-7-17(8-4-1)11-14-21-15-12-19(13-16-21)20-18-9-5-2-6-10-18/h1-10,19-20H,11-16H2/i2+1,5+1,6+1,9+1,10+1,18+1InChi KeyZCMDXDQUYIWEKB-WGIVQRENSA-N Order 4-Anilino-N-phenethylpiperidine (4-ANPP) PrecursorRegulatory Information
DEA ScheduleShipping & Storage Information
Storage-20°CShipping Room Temperature in continental US; may vary elsewhereStability ≥ 73 monthsBuy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
Buy 4-Anilino-N-phenethylpiperidine (4-ANPP)
1.Pease, J.P., LePine, A.J., and Smith, C.M.Methods for preparing fentanyl and fentanyl intermediatesPatent Application PublicationUS 2013/0281702 A1(2013) 2.Watanabe, S., Vikingsson, S., Roman, M., et al.In vitro and in vivo metabolite identification studies for the new synthetic opioids acetylfentanyl, acrylfentanyl, furanylfentanyl, and 4-fluoro-isobutyrylfentanylAAPS J.19(4)1102-1122(2017) 3.Labroo, R.B., Paine, M.F., Thummel, K.E., et al.Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: Implications for interindividual variability in disposition, efficacy, and drug interactionsDrug Metab. Dispos.25(9)1072-1079(1997) 4.Melent’ev, A.B., Kataev, S.S., and Dvorskaya, O.N.Identification and analytical properties of acetyl fentanyl metabolitesJ. Anal. Chem.70(2)216-224(2015) 5.Steuer, A.E., Williner, E., Staeheli, S.N., et al.Studies on the metabolism of the fentanyl-derived designer drug butyrfentanyl in human in vitro liver preparations and authentic human samples using liquid chromatography-high resolution mass spectrometry (LC-HRMS)Drug Test Anal.9(7)1085-1092(2017)
4-ANPP (Item No. 18810) is an analytical reference material categorized as an opioid metabolite and a precursor in the synthesis of fentanyl (Item Nos. ISO60197 | 22659 | 14719) and other opioids. 1, 2, 3, 4, 5 4-ANPP is a metabolite of acetyl fentanyl (Item Nos. ISO60128 | ISO00128), butyryl fentanyl (Item Nos. 19734 | 14728), furanyl fentanyl (Item Nos. 19633 | 18705), acrylfentanyl (Item Nos. 23060 | 19312), and fentanyl. 1, 2, 3, 4 It has also been found as an impurity in illicit fentanyl preparations. 6 4-ANPP is regulated as a Schedule II compound in the United States.
4-ANPP (N-phenyl-1- (2-phenylethyl) -4-piperidinamine), a new opioid drug, classified as piperidinamine, synthesized as an intermediate between fentanyl from N-phenethyl-4-piperidone. 4-ANPP is a metabolite of acetylfentanil, butyrylfentanyl, furanylfentanil, acrylfentanyl and fentanyl. 4-ANPP is produced on modern chemical equipment, by professionals who know their business, which makes it clean and of high quality.
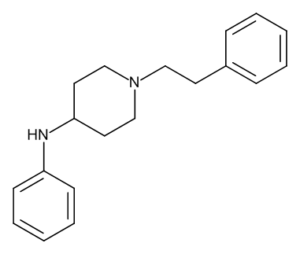
4-ANPP Synonyms - 4-ANPP
- 4-Aminophenyl-1-phenethylpiperidine
- 4-Anilino-N-phenethylpiperidine
- Despropionyl fentanyl
IUPAC N-phenyl-1-(2-phenylethyl)-4-piperidinamine Formula C19H24N2 Molecular weight 280.4 g/mol CAS 21409-26-7 Appearance Powder Purity ≥ 98% 4-ANPP and other chemicals sold on this website are designed and must be used strictly for research and forensic medical examination.
Adverse properties of have not been studied.
Storage conditions of this chemical: in a cool and dry place. The stability of this chemical compound can last up to 2 years, under the right storage conditions.
CAS 21409-26-7












Reviews
There are no reviews yet.