Buy Tyramine Cas 51-67-2
Tyramine (/ˈtaɪrəmiːn/ TY-rə-meen) (also spelled tyramin), also known under several other names,[note 1] is a naturally occurring trace amine derived from the amino acid tyrosine.[4] Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs).
Occurrence
Tyramine occurs widely in plants[5] and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date.
Specific foods containing considerable amounts of tyramine include:[6][7]
- Strong or aged cheeses: cheddar, Swiss, Parmesan, Stilton, Gorgonzola or blue cheeses, Camembert, feta, Muenster
- Meats that are cured, smoked, or processed: such as salami, pepperoni, dry sausages, hot dogs, bologna, bacon, corned beef, pickled or smoked fish, caviar, aged chicken livers, soups or gravies made from meat extract
- Pickled or fermented foods: sauerkraut, kimchi, tofu (especially stinky tofu), pickles, miso soup, bean curd, tempeh, sourdough breads
- Condiments: soy, shrimp, fish, miso, teriyaki, and bouillon-based sauces
- Drinks: beer (especially tap or home-brewed), vermouth, red wine, sherry, liqueurs
- Beans, vegetables, and fruits: fermented or pickled vegetables, overripe fruits
- Chocolate[8]
Scientists more and more consider tyramine in food as an aspect of safety.[9] They propose projects of regulations aimed to enact control of biogenic amines in food by various strategies, including usage of proper fermentation starters, or preventing their decarboxylase activity.[10] Some authors wrote that this has already given positive results, and tyramine content in food is now lower than it has been in the past.[11]
In plants
Mistletoe (toxic and not used by humans as a food, but historically used as a medicine).[12]
In animals
Tyramine also plays a role in animals including: In behavioral and motor functions in Caenorhabditis elegans;[13] Locusta migratoria swarming behaviour;[14] and various nervous roles in Rhipicephalus, Apis, Locusta, Periplaneta, Drosophila, Phormia, Papilio, Bombyx, Chilo, Heliothis, Mamestra, Agrotis, and Anopheles.[15]
Biological activity
| Compound | NETooltip Norepinephrine | DATooltip Dopamine | 5-HTTooltip Serotonin | Ref |
|---|---|---|---|---|
| Phenethylamine | 10.9 | 39.5 | >10,000 | [16][17][18] |
| Tyramine | 40.6 | 119 | 2,775 | [19][18] |
| Dopamine | 66.2 | 86.9 | >10,000 (RI) | [19][18] |
| Norepinephrine | 164 | 869 | >10,000 | [19][18] |
| Tryptamine | 716 | 164 | 32.6 | [20][21] |
| Dextroamphetamine | 6.6–7.2 | 5.8–24.8 | 698–1,765 | [19][22] |
| Notes: The smaller the value, the more strongly the drug releases the neurotransmitter. The assays were done in rat brain synaptosomes and human potencies may be different. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs:[23][24] | ||||
Tyramine is a norepinephrine and dopamine releasing agent (NDRA) and indirectly acting sympathomimetic.[19][18] Evidence for the presence of tyramine in the human brain has been confirmed by postmortem analysis.[25] Additionally, the possibility that tyramine acts directly as a neuromodulator was revealed by the discovery of a G protein-coupled receptor with high affinity for tyramine, called the trace amine-associated receptor (TAAR1).[26][27] The TAAR1 receptor is found in the brain, as well as peripheral tissues, including the kidneys.[28] Tyramine is a full agonist of the TAAR1 in rodents and humans.[29][30]
Tyramine is physiologically metabolized by monoamine oxidases (primarily MAO-A), FMO3, PNMT, DBH, and CYP2D6.[31][32][33][34][35] Human monoamine oxidase enzymes metabolize tyramine into 4-hydroxyphenylacetaldehyde.[36] If monoamine metabolism is compromised by the use of monoamine oxidase inhibitors (MAOIs) and foods high in tyramine are ingested, a hypertensive crisis can result, as tyramine also can displace stored monoamines, such as dopamine, norepinephrine, and epinephrine, from pre-synaptic vesicles. Tyramine is considered a “false neurotransmitter“, as it enters noradrenergic nerve terminals and displaces large amounts of norepinephrine, which enters the blood stream and causes vasoconstriction.
Additionally, cocaine has been found to block blood pressure rise that is originally attributed to tyramine, which is explained by the blocking of adrenaline by cocaine from reabsorption to the brain.[37]
The first signs of this effect were discovered by a British pharmacist who noticed that his wife, who at the time was on MAOI medication, had severe headaches when eating cheese.[38] For this reason, it is still called the “cheese reaction” or “cheese crisis”, although other foods can cause the same problem.[39]
Most processed cheeses do not contain enough tyramine to cause hypertensive effects, although some aged cheeses (such as Stilton) do.[40][41]
A large dietary intake of tyramine (or a dietary intake of tyramine while taking MAO inhibitors) can cause the tyramine pressor response, which is defined as an increase in systolic blood pressure of 30 mmHg or more. The increased release of norepinephrine (noradrenaline) from neuronal cytosol or storage vesicles is thought to cause the vasoconstriction and increased heart rate and blood pressure of the pressor response. In severe cases, adrenergic crisis can occur.[medical citation needed] Although the mechanism is unclear, tyramine ingestion also triggers migraine attacks in sensitive individuals and can even lead to stroke.[42] Vasodilation, dopamine, and circulatory factors are all implicated in the migraines. Double-blind trials suggest that the effects of tyramine on migraine may be adrenergic.[43]
Research reveals a possible link between migraines and elevated levels of tyramine. A 2007 review published in Neurological Sciences[44] presented data showing migraine and cluster diseases are characterized by an increase of circulating neurotransmitters and neuromodulators (including tyramine, octopamine, and synephrine) in the hypothalamus, amygdala, and dopaminergic system. People with migraine are over-represented among those with inadequate natural monoamine oxidase, resulting in similar problems to individuals taking MAO inhibitors. Many migraine attack triggers are high in tyramine.[45]
If one has had repeated exposure to tyramine, however, there is a decreased pressor response; tyramine is degraded to octopamine, which is subsequently packaged in synaptic vesicles with norepinephrine (noradrenaline).[citation needed] Therefore, after repeated tyramine exposure, these vesicles contain an increased amount of octopamine and a relatively reduced amount of norepinephrine. When these vesicles are secreted upon tyramine ingestion, there is a decreased pressor response, as less norepinephrine is secreted into the synapse, and octopamine does not activate alpha or beta adrenergic receptors.[medical citation needed]
When using a MAO inhibitor (MAOI), an intake of approximately 10 to 25 mg of tyramine is required for a severe reaction, compared to 6 to 10 mg for a mild reaction.[46]
Tyramine, like phenethylamine, is a monoaminergic activity enhancer (MAE) of serotonin, norepinephrine, and dopamine in addition to its catecholamine-releasing activity.[47][48][49] That is, it enhances the action potential-mediated release of these monoamine neurotransmitters.[47][48][49] The compound is active as a MAE at much lower concentrations than the concentrations at which it induces the release of catecholamines.[47][48][49] The MAE actions of tyramine and other MAEs may be mediated by TAAR1 agonism.[50][51]
Biosynthesis
Biochemically, tyramine is produced by the decarboxylation of tyrosine via the action of the enzyme tyrosine decarboxylase.[52] Tyramine can, in turn, be converted to methylated alkaloid derivatives N-methyltyramine, N,N-dimethyltyramine (hordenine), and N,N,N-trimethyltyramine (candicine).
-
Tyramine
-
N-Methyltyramine
-
N,N-Dimethyltyramine (hordenine)
-
N,N,N-Trimethyltyramine (candicine)
In humans, tyramine is produced from tyrosine, as shown in the following diagram.
|
p-Tyramine
In humans, catecholamines and phenethylaminergic trace amines are derived from the amino acid L-phenylalanine. |
Chemistry
In the laboratory, tyramine can be synthesized in various ways, in particular by the decarboxylation of tyrosine.[53][54][55]

Society and culture
51-67-2
-
-
Basic information
- Product Name: Tyramine
- Synonyms: 4-(2-aminoethyl)-pheno;4-(2-Aminoethyl)-phenol (thyramin);4-hydroxy-benzeneethanamin;4-Hydroxy-beta-phenylethylamine;alpha-(4-Hydroxyphenyl)-beta-aminoethane;Benzeneethanamine, 4-hydroxy-;beta-(4-Hydroxyphenyl)ethylamine;beta-Hydroxyphenylethylamine
- CAS NO:51-67-2
- Molecular Formula: C8H11NO
- Molecular Weight: 137.17904
- EINECS: 200-115-8
- Product Categories: Anilines, Aromatic Amines and Nitro Compounds;Amines;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals
- Mol File: 51-67-2.mol
-
Chemical Properties
- Melting Point: 160-162 °C(lit.)
- Boiling Point: 175-181 °C8 mm Hg(lit.)
- Flash Point: 165°C
- Appearance: Clear yellow to orange to slightly brown, may darken on storage/Liquid
- Density: 1.0630 (rough estimate)
- Vapor Pressure: 0.000123mmHg at 25°C
- Refractive Index: 1.5849 (estimate)
- Storage Temp.: Refrigerator, Under Inert Atmosphere
- Solubility: DMSO (Slightly), Methanol (Slightly)
- PKA: 9.74(at 25℃)
- Water Solubility: 1g/95mL (15 ºC)
- Stability: Stable. Incompatible with strong acids, strong oxidizing agents.
- Merck: 14,9835
- BRN: 1099914
- CAS DataBase Reference: Tyramine(CAS DataBase Reference)
- NIST Chemistry Reference: Tyramine(51-67-2)
- EPA Substance Registry System: Tyramine(51-67-2)
- Product Description:Product Name: tyramine CAS NO: 51-67-2
Synonyms:
4-(2-aminoethyl)-pheno;
2-(4-Hydroxyphenyl)ethylamine;
4-hydroxy-phenethylamine;
Chemical & Physical Properties:
Appearance: Yellow to brown crystals
Assay :≥98.0%
Density: 1.103g/cm3
Boiling Point: 175-181℃ (8 mmHg)
Melting Point: 155-163℃
Flash Point: 165℃
Water Solubility: 1g/95mL (15℃)
Stability: Stable. Incompatible with strong acids, strong oxidizing agents.
Storage Condition: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Safety Information:
RTECS: SJ5950000
Safety Statements: S26-S37/39
HS Code: 2922299090
WGK Germany: 3
Risk Statements: R36/37/38
Hazard Code: Xi
Symbol: GHS07
Signal Word: Warning
Hazard Statements: H315-H319-H335
Precautionary Statements: P261-P305 + P351 + P338
Tyramine, also known by several other names, is a naturally occurring monoamine and trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with monoamine oxidase inhibitors (MAOIs).
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Hot Tags: tyramine cas 51-67-2, manufacturers, suppliers, factory, buy, in stock, CAS 768341 84 0, Salmeterol Hydroxynapthoate, CAS 376591 94 5, CAS 50 02 2, 4 Fluoro 2 methoxyaniline, CAS 2136287 66 4
Structure
2D Structure
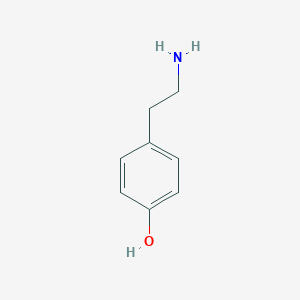
3D Structure
Properties
IUPAC Name |
4-(2-aminoethyl)phenol | |
|---|---|---|
InChI |
InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | |
InChI Key |
DZGWFCGJZKJUFP-UHFFFAOYSA-N | |
Canonical SMILES |
C1=CC(=CC=C1CCN)O | |
Molecular Formula |
C8H11NO | |
Related CAS |
71495-67-5 | |
DSSTOX Substance ID |
DTXSID2043874 | |
Molecular Weight |
137.18 g/mol | |
Physical Description |
Solid, Colourless to yellow solid; Sweet meaty aroma | |
Boiling Point |
166 °C @ 2 MM HG | |
Solubility |
SOL IN WATER /TYRAMINE HYDROCHLORIDE/, 1 G DISSOLVES IN 10 ML BOILING ALCOHOL, SPARINGLY SOL IN BENZENE, XYLENE, Slightly soluble in water, benzene; soluble in ethanol, xylene, Water solubility = 10.4E+3 mg/L @ 15 °C, 10.4 mg/mL at 15 °C, Soluble in water, Soluble (in ethanol) | |
Color/Form |
CRYSTALS FROM BENZENE OR ALCOHOL, PLATES OR NEEDLES FROM BENZENE, NEEDLES FROM WATER | |
CAS No. |
51-67-2 | |
Melting Point |
164-165 °C, MP: 269 °C /TYRAMINE HYDROCHLORIDE/, 164 – 165 °C |
Foundational & Exploratory
An In-depth Technical Guide to the Tyramine Biosynthesis Pathway in Microbial Organisms
A Historical Perspective on the Discovery of Tyramine: An In-depth Technical Guide
tyramine as a neuromodulator versus a classical neurotransmitter
An In-depth Technical Guide to the Physicochemical Properties and Stability of Tyramine in Solution
Characterization of Novel Tyramine Receptors and Their Signaling Cascades: An In-depth Technical Guide
The Enzymatic Degradation and Metabolic Fate of Tyramine: A Technical Guide
Genetic Determinants of Tyramine Production in Fermented Foods: A Technical Guide
Methodological & Application
Application Note: HPLC-Based Quantification of Tyramine in Cheese and Wine Samples
Standardized Protocol for Tyramine Extraction from Neuronal Tissue: Application Notes
Application Notes and Protocols for Conducting a Tyramine Challenge Test to Assess Monoamine Oxidase Inhibitor (MAOI) Safety
Application Notes and Protocols: Tyramine as a Pharmacological Probe for Monoamine Oxidase Activity
Application Notes and Protocols for In Vivo Microdialysis Monitoring of Synaptic Tyramine
Application Notes and Protocols for Carbon-14 Labeled Tyramine in Binding Studies
Development of High-Throughput Screening Assays for Tyramine Receptor Ligands
analyzing urinary tyramine as a potential biomarker for metabolic disorders
Application of Tyramine-Based Enzymatic Biosensors for Food Safety Testing
Application Notes and Protocols: Utilization of Tyramine in the Development of Targeted Neurological Drug Delivery
Troubleshooting & Optimization
overcoming matrix interference in electrochemical detection of tyramine
optimizing chromatographic separation of tyramine from other biogenic amines
addressing challenges in the quantification of trace levels of tyramine
best practices for ensuring tyramine stability in long-term sample storage
troubleshooting unexpected mortality in animal models of tyramine-induced hypertension










Reviews
There are no reviews yet.