Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
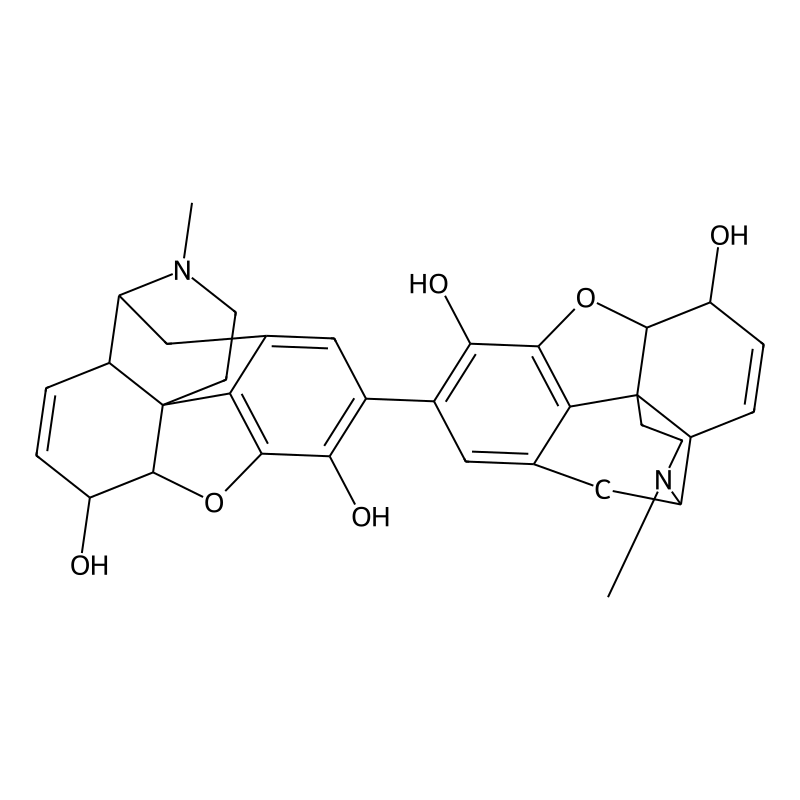
Pseudomorphine (also known as oxydimorphine or dehydromorphine) is an inactive, natural dimerisation product of the morphine molecule in tandem and thus a common impurity in morphine concentrations. It was first described by Pelletier in 1835.[2]
This compound may be synthesized by the oxidative coupling of morphine by potassium ferricyanide.[1]
Pseudomorphine contributes very little to morphine’s effects. It produces no effects in the central nervous or gastrointestinal systems, but it might have some effects on the circulatory system. Buy Pseudomorphine oxydimorphine Cas-125-24-6
See also
- Thebaine (paramorphine)
- Morphine-N-oxide
- Morphine-3-glucuronide
- Morphine-6-glucuronide
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Buy Pseudomorphine oxydimorphine Cas-125-24-6
Chemical and Physical Properties
Oxydimorphine is characterized by its molecular formula and weight, reflecting its dimeric nature. Its physical state and solubility are also important parameters for its handling and analysis.
Table 2: Key of this compound
| Property | Value | Source Index |
| Chemical Formula | C34H36N2O6 | |
| Molecular Weight | 568.670 g/mol | |
| 568.67 g/mol | ||
| 568.66 g/mol | ||
| 568.7 g/mol | ||
| 568.25734 | ||
| CAS Number | 125-24-6 | |
| Melting Point | >225 °C (dec.) | |
| >215 °C | ||
| Density | 1.58 g/cm³ | |
| LogP | 2.25240 | |
| XLogP3 | 2.8 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 1 | |
| Heavy Atom Count | 42 | |
| Exact Mass | 568.25733687 | |
| Polar Surface Area (PSA) | 105.86000 |
Compound List:
this compound
Pseudomorphine
Dehydromorphine
2,2′-Bimorphine
Morphine
Synthetic Methodologies and Mechanistic Investigations of Oxydimorphine Formation
Chemical Synthesis of Oxydimorphine via Oxidative Dimerization of Morphine
The formation of this compound, also known as pseudomorphine or 2,2′-bimorphine, from morphine is a classic example of oxidative dimerization. This process involves the coupling of two morphine molecules at the C-2 position of each aromatic ring. The semi-systematic name 2,2′-bimorphine is particularly descriptive as it specifies the points of union between the two morphine units.
The synthesis is typically achieved by treating a solution of a morphine salt, such as the hydrochloride, with an oxidizing agent. The use of a morphine salt is crucial due to its significantly higher water solubility compared to the parent morphine molecule, which is practically insoluble. For instance, morphine hydrochloride and sulfate (B86663) salts are about 300 times more water-soluble than morphine itself.
Mild oxidizing agents are instrumental in facilitating the dimerization of morphine to this compound. Agents like potassium permanganate (B83412) (KMnO4) and alkaline ferricyanide (B76249) are commonly employed for this transformation.
Potassium Permanganate (KMnO₄): In a reaction described by Beckurt, a 0.1 N solution of potassium permanganate is used to treat a solution of morphine hydrochloride, leading to the precipitation of white this compound. The permanganate ion (MnO₄⁻) acts as the oxidant, accepting an electron from the morphine molecule and being reduced to the manganate (B1198562) ion (MnO₄²⁻). This one-electron transfer is a key step in initiating the dimerization process.
Alkaline Ferricyanide [K₃Fe(CN)₆]: The oxidation of morphine to 2,2′-bimorphine can also be achieved using alkaline ferricyanide. This reaction is known to proceed through the formation of a mesomeric aryloxy free radical, which then leads to the dimer. The determination of morphine can be accomplished using potassium ferricyanide through the formation of pseudomorphine.
The primary function of these mild oxidizing agents is to abstract an electron from the morphine molecule, thereby generating a reactive intermediate that can undergo coupling. Stronger oxidizing agents might lead to over-oxidation and the formation of undesired byproducts.
The efficiency and outcome of the oxidative dimerization of morphine are significantly influenced by various reaction conditions.
pH: The reaction is often carried out in an aqueous medium. The pH of the solution plays a critical role, particularly in the formation of the reactive species from morphine. In an alkaline medium, the phenolic group at C-3 of morphine is more readily deprotonated, which enhances its electron-donating ability and facilitates the subsequent steps of the reaction. Even in a neutral aqueous solution, the weak basicity of water can lead to the formation of a phenoxide ion. However, the formation of the crucial carbanion intermediate at C-2 is described as being independent of an acidic medium.
Solvents: The choice of solvent is primarily dictated by the solubility of the reactants. As morphine itself has very low water solubility, its more soluble salts, like the hydrochloride, are used in aqueous solutions. The use of water as a solvent can also play a role in the reaction mechanism by acting as a weak base to deprotonate the phenolic hydroxyl group of morphine.
Temperature: While specific temperature data for the permanganate-induced dimerization is not extensively detailed in the provided context, reaction temperatures are a general factor in controlling reaction rates. For many organic reactions, including those involving oxidation, temperature can affect the kinetics of the reaction and the stability of intermediates. In some syntheses, elevated temperatures are used to increase reaction rates, though this can sometimes lead to side reactions.
Table 1: Influence of Reaction Conditions on this compound Synthesis
Reaction Mechanisms of this compound Formation
The formation of this compound from morphine is a complex process involving several key mechanistic steps, including the generation of radical and carbanionic intermediates and redox processes.
A central feature of this compound formation is the coupling of two morphine-derived radicals. The process is initiated by a one-electron oxidation of the morphine molecule by an oxidizing agent like permanganate. This electron abstraction generates a free radical species.
The stabilization of this radical is achieved through coupling, where two of these radical intermediates combine to form a new carbon-carbon bond between the C-2 positions of the two morphine units, yielding the dimeric structure of this compound. This radical-radical coupling is a termination step that results in a stable, non-radical product. The activation energy for the coupling of carbon-centered radicals is nearly zero, making this a very fast and efficient process.
The phenolic hydroxyl group at the C-3 position of morphine plays a pivotal role in directing the dimerization to the C-2 position. The electron-donating nature of this phenolic group is crucial.
In the presence of a weak base like water, the phenolic group can deprotonate to form a phenoxide ion. The negative charge of the phenoxide ion can be delocalized through resonance into the aromatic ring. This resonance stabilization leads to an increase in electron density at the ortho and para positions. Specifically, it facilitates the formation of a carbanion at the C-2 position. This carbanion at C-2 is a highly reactive intermediate. It is this carbanion that then participates in the redox reaction with the oxidizing agent.
The process can be summarized as follows:
Formation of a Reactive Intermediate: The phenolic group at C-3 facilitates the formation of a carbanion at C-2.
Electron Transfer: This C-2 carbanion intermediate transfers a single electron to the permanganate ion (Mn⁷⁺O₄⁻). This is an electron transfer or outer-sphere mechanism.
Oxidation and Reduction: The carbanion is oxidized to a free radical, and the permanganate is reduced to the manganate ion (Mn⁶⁺O₄²⁻).
Radical Coupling: The resulting free radical on the morphine molecule is stabilized by coupling with another identical radical, forming the 2,2′-dimer, this compound.
The reaction likely proceeds through an intermediate enol which then tautomerizes to the final ketone structure within the cyclohexene (B86901) ring of the morphine skeleton. The two resulting bulky ketone groups in the dimer are arranged in a transoid fashion to minimize steric repulsion.
Table 2: Key Mechanistic Steps in this compound Formation
Alternative Synthetic Routes and Precursors
The synthesis of this compound, also known as pseudomorphine or 2,2′-bimorphine, is predominantly achieved through the oxidative dimerization of its direct precursor, morphine. The literature describes several alternative methods that primarily vary in the choice of oxidizing agent and reaction conditions. These routes leverage the phenolic hydroxyl group at the C-3 position of the morphine molecule, which facilitates the coupling reaction.
The formation of this compound via the oxidation of morphine can be accomplished using a variety of mild oxidizing agents. Historically and in laboratory settings, reagents such as potassium permanganate and potassium ferricyanide have been effectively employed. The reaction is typically conducted in a mildly alkaline medium, for example, in the presence of sodium bicarbonate, to facilitate the process.
Mechanistic studies, particularly concerning the use of potassium permanganate, provide insight into the formation of the dimer. A theoretical study of Beckurt’s test for alkaloids, which uses potassium permanganate, proposes a mechanism initiated by the enhancement of the electron donor property of the phenol (B47542) group in morphine. This is achieved through a reaction with a weak base like water, leading to the formation of a phenoxy ion. Resonance stabilization of this ion generates a carbanion at the C-2 position of the morphine molecule. This carbanion then transfers an electron to the permanganate ion, resulting in its reduction and the concomitant oxidation of the morphine molecule to a free radical. The final step is the coupling of these radicals to yield 2,2′-bimorphine (this compound). For this test, a salt of the alkaloid, such as morphine hydrochloride, is often used due to its greater solubility compared to the parent base.
Other oxidizing agents have also been investigated for the transformation of morphine. The use of lead superoxide (B77818) (lead dioxide) in a sulfuric acid solution has been reported to yield oxidation products of morphine. Similarly, the interaction of morphine with antimony trichloride, which is hydrolyzed in situ to antimony oxychloride, can lead to the formation of an o-benzoquinone derivative, indicating the reactivity of the phenolic ring towards oxidation.
The following table summarizes various reported methods for the synthesis of this compound from morphine, highlighting the different precursors (in the form of salts) and oxidizing systems used.
| Precursor | Oxidizing Agent | Reaction Medium/Conditions | Reference(s) |
| Morphine | Potassium permanganate | Mildly alkaline (e.g., Sodium bicarbonate) | |
| Morphine | Potassium ferricyanide | Mildly alkaline (e.g., Sodium bicarbonate) | |
| Morphine hydrochloride | Potassium permanganate (0.1 N solution) | Aqueous solution | |
| Morphine | Lead superoxide (Lead dioxide) | Sulfuric acid (1/20 N) |
Advanced Analytical Approaches for Oxydimorphine Detection and Quantification
Chromatographic Separation Techniques for Oxydimorphine
Chromatography is a cornerstone of analytical chemistry, enabling the separation of individual components from a mixture. For this compound and related compounds, several chromatographic techniques are utilized, each offering distinct advantages in terms of resolution, speed, and applicability to different sample types.
High-Performance Liquid Chromatography (HPLC) is a widely used technique for the analysis of opioids due to its versatility in handling non-volatile and thermally labile compounds like this compound. The separation is typically achieved on reversed-phase columns, such as C18 or PFP (Pentafluorophenyl), which provide effective retention and resolution of polar analytes. The mobile phase often consists of a mixture of an aqueous buffer (e.g., ammonium (B1175870) formate (B1220265) or formic acid in water) and an organic solvent like acetonitrile (B52724) or methanol, run in a gradient elution mode to optimize separation.
Various detection modes can be coupled with HPLC for the analysis of this compound:
Ultraviolet (UV) Detection: UV detectors are commonly used in HPLC. For this compound, detection is typically performed at a wavelength around 280 nm. While robust and widely available, UV detection may lack the sensitivity required for trace-level analysis in biological samples.
Electrochemical Detection (ED): This method offers higher sensitivity for electrochemically active compounds like morphine and can be applied to this compound. It involves applying a potential and measuring the current generated by the oxidation of the analyte.
Fluorescence Detection: Although less common for this compound itself unless derivatized, fluorescence detection is a highly sensitive technique used for some opioids.
Mass Spectrometry (MS) Detection: The coupling of HPLC with MS is the most powerful approach, providing both separation and definitive identification. This is discussed in detail in section 4.2.1.
| Parameter | Condition 1 | Condition 2 |
| Column | Kinetix biphenyl (B1667301) (2.1 x 100 mm, 1.7 µm) | AccuCore PFP |
| Mobile Phase A | 5 mM Ammonium formate in 0.1% aqueous formic acid | Water with 0.1% formic acid |
| Mobile Phase B | 100% Methanol | Methanol with 0.1% formic acid |
| Flow Rate | 0.4 mL/min | Not Specified |
| Detection | MS/MS | MS/MS |
Gas Chromatography (GC) is another powerful separation technique, but it is generally suitable for volatile and thermally stable compounds. This compound, like other opiates, is not inherently volatile due to the presence of polar hydroxyl and ketone functional groups. Therefore, a derivatization step is necessary to convert it into a more volatile and thermally stable form suitable for GC analysis.
The derivatization process typically involves two steps:
Oximation: The ketone group on this compound is converted to a methoxime using reagents like methoxyamine.
Acylation: The hydroxyl groups are converted to esters (e.g., propionyl esters) using an anhydride (B1165640) like propionic anhydride.
After derivatization, the resulting volatile compound can be separated on a GC column and detected, most commonly by mass spectrometry (GC-MS). This method allows for the simultaneous analysis of several opiates in a single run. The limit of quantitation for oxymorphone using GC-MS has been reported to be as low as 10 ng/mL in blood and 25 ng/mL in urine.
Supercritical Fluid Chromatography (SFC) is a hybrid of gas and liquid chromatography that uses a supercritical fluid, most commonly carbon dioxide (CO2), as the mobile phase. SFC has gained renewed interest due to advancements in instrumentation that have addressed earlier limitations in reliability and quantitative performance. This technique is particularly valuable for the analysis of complex mixtures and offers a separation mechanism that is orthogonal to reversed-phase HPLC.
Key features of SFC for opioid analysis include:
High Efficiency and Speed: The low viscosity and high diffusivity of supercritical fluids allow for faster separations and shorter analysis times compared to HPLC. A method for analyzing 19 different opioids in less than two minutes has been demonstrated.
Green Chemistry: The primary use of CO2, a non-toxic and environmentally benign solvent, reduces the consumption of organic solvents.
Versatility: By adding a polar organic co-solvent (modifier), such as methanol, to the CO2 mobile phase, SFC can be used to analyze a wide range of compounds, from non-polar to polar. The technique is well-suited for both chiral and achiral separations in pharmaceutical analysis.
Mass Spectrometry-Based Identification and Quantification
Mass Spectrometry (MS) is an analytical technique that measures the mass-to-charge ratio (m/z) of ions. It is an indispensable tool for the definitive identification and sensitive quantification of compounds like this compound, especially when coupled with a chromatographic separation technique.
The combination of Liquid Chromatography with Mass Spectrometry (LC-MS) or tandem Mass Spectrometry (LC-MS/MS) is the gold standard for the quantification of this compound in biological matrices such as plasma, oral fluid, and wastewater. This approach offers unparalleled sensitivity and selectivity, allowing for detection at very low concentrations.
In a typical LC-MS/MS workflow, samples undergo preparation, often involving protein precipitation or solid-phase extraction (SPE), to remove interferences. An isotopically labeled internal standard (e.g., oxymorphone-d3) is added to correct for matrix effects and variations in extraction recovery.
The mass spectrometer is usually operated with an electrospray ionization (ESI) source in the positive ion mode. For quantification, the most selective mode is Multiple Reaction Monitoring (MRM), where a specific precursor ion for this compound is selected and fragmented, and one or more specific product ions are monitored. This process ensures high specificity, as it is unlikely that another compound will have the same retention time, precursor ion mass, and product ion masses. LC-MS/MS methods have been developed to quantify oxymorphone with lower limits of quantification (LLOQ) in the sub-ng/mL range.
| Compound | Precursor Ion (m/z) | Product Ion 1 (m/z) | Product Ion 2 (m/z) |
| Oxymorphone | 302.1 | 227.1 | 284.1 |
| Oxycodone | 316.1 | 298.1 | 241.1 |
| Hydromorphone | 286.1 | 185.0 | 157.0 |
| Morphine | 286.1 | 165.0 | 201.0 |
| Codeine | 300.1 | 165.0 | 215.1 |
| Hydrocodone | 300.1 | 199.1 | 171.1 |
Note: Data compiled from various sources for illustrative purposes. Actual values may vary based on instrumentation and conditions.
Ion Mobility Spectrometry (IMS) is a gas-phase separation technique that separates ions based on their size, shape, and charge. When coupled with mass spectrometry (IMS-MS), it provides an additional dimension of separation, enhancing analytical specificity. This is particularly useful for resolving isomers—compounds with the same mass but different structures—which cannot be distinguished by MS alone.
In IMS-MS, ions are passed through a drift tube filled with a buffer gas under the influence of a weak electric field. Ions with a more compact structure will travel faster than more extended ions of the same mass-to-charge ratio. The resulting drift time can be used to calculate a collisional cross section (CCS), which is a characteristic physical property of the ion.
Recent studies have demonstrated the power of Drift Tube Ion Mobility Spectrometry-Mass Spectrometry (DTIMS-MS) and Trapped Ion Mobility Spectrometry (TIMS) for the analysis of opioids. These techniques can differentiate between isomeric opioids without prior chromatographic separation, enabling high-throughput screening of samples in as little as 10 seconds per sample. The combination of retention time (from LC), CCS (from IMS), and accurate mass (from MS) provides extremely high confidence in compound identification.
Spectroscopic Methods for Detection and Characterization
Spectroscopic techniques are fundamental in the analytical sciences for the identification and quantification of chemical compounds. These methods rely on the interaction of electromagnetic radiation with the analyte. For a compound like this compound, various spectroscopic approaches can be employed for both preliminary screening and detailed characterization.
UV-Visible Spectrophotometry (e.g., Color Tests)
A significant application of UV-Vis spectrophotometry in forensic and preliminary analysis is through color tests. These tests are simple, rapid, and cost-effective presumptive methods that indicate the possible presence of a particular class of compounds through a color change. They involve mixing the sample with a specific chemical reagent and observing the resulting color. While these tests are generally not specific to a single compound, they are effective for screening and narrowing down possibilities. For opioids, several classic colorimetric reagents are used. The reaction of an unknown sample with these reagents can provide a tentative identification, which must then be confirmed by more specific analytical methods.
| Reagent | Composition | Typical Color Reaction with Opioids (e.g., Morphine, Heroin) |
|---|---|---|
| Marquis Reagent | Formaldehyde in concentrated Sulfuric Acid | Purple to violet |
| Froehde’s Reagent | Molybdic acid in concentrated Sulfuric Acid | Purple, turning to green then blue |
| Mecke Reagent | Selenious acid in concentrated Sulfuric Acid | Green to blue-green |
This table summarizes common colorimetric tests used for the presumptive identification of opioids. The specific color and intensity can vary based on the specific opioid and its concentration.
Immunoanalytical Techniques (e.g., ELISA) for Targeted Detection
Immunoanalytical techniques leverage the highly specific binding interaction between an antibody and its target antigen. Among these methods, the Enzyme-Linked Immunosorbent Assay (ELISA) is a widely used platform for detecting and quantifying substances such as drugs and their metabolites in biological samples like urine, blood, or oral fluid.
ELISAs for opioid detection typically operate on a competitive binding principle. In this format, the sample (potentially containing the drug) is mixed with a known quantity of enzyme-labeled drug (conjugate). This mixture is then added to microplate wells that are pre-coated with antibodies specific to the target drug class. The drug in the sample and the enzyme-labeled drug conjugate compete for the limited number of antibody binding sites. After an incubation period, the wells are washed to remove any unbound material. A substrate is then added, which reacts with the enzyme on the bound conjugate to produce a measurable color change. The intensity of the color is inversely proportional to the concentration of the drug in the original sample; a lower color intensity indicates a higher drug concentration.
These assays are designed as screening tests and are valued for their sensitivity and ability to process many samples at once. However, a key performance characteristic of any immunoassay is its specificity, or its ability to distinguish between different but structurally related compounds. This is often described in terms of cross-reactivity. An antibody may bind to other related molecules, which can lead to a positive result for a class of compounds rather than a single substance. For example, an opiate ELISA may show varying degrees of reactivity with morphine, codeine, and other related structures. Any presumptive positive result from an ELISA test requires confirmation by a more specific method like gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS).
| Compound | Cross-Reactivity (%) |
|---|---|
| Oxycodone | 100% |
| Oxymorphone | 85% |
| Hydrocodone | 35% |
| Codeine | 12% |
| Hydromorphone | 10% |
| Morphine | <1% |
This table presents representative cross-reactivity data for an Oxycodone/Oxymorphone ELISA kit. Cross-reactivity is determined by identifying the concentration of a related compound that produces a response equivalent to a specific concentration of the primary target (e.g., oxycodone). This illustrates the variable specificity of immunoassays for structurally similar compounds. Data is illustrative and based on commercially available kit information.
Method Validation and Performance Characteristics (Specificity, Sensitivity, Accuracy)
The validation of an analytical method is a critical process that demonstrates its suitability for its intended purpose. International guidelines, such as those from the International Council for Harmonisation (ICH), outline the key performance characteristics that must be evaluated. These include specificity, sensitivity, and accuracy.
Specificity is the ability of the method to assess the analyte unequivocally in the presence of other components that may be expected to be present, such as impurities, degradation products, or matrix components. For chromatographic methods like LC-MS/MS, specificity is demonstrated by showing that the analyte’s peak is well-resolved from other components and that no interfering peaks are present in blank samples.
Sensitivity refers to the method’s ability to detect and/or quantify low concentrations of the analyte. It is typically defined by the Limit of Detection (LOD) and the Limit of Quantitation (LOQ). The LOD is the lowest concentration of an analyte in a sample that can be reliably detected, but not necessarily quantified with acceptable precision and accuracy. The LOQ is the lowest concentration that can be determined with acceptable precision and accuracy under the stated experimental conditions.
Accuracy represents the closeness of the test results obtained by the method to the true value. It is often assessed by analyzing samples with known concentrations of the analyte (e.g., spiked control samples) and comparing the measured value to the theoretical value. Accuracy is typically expressed as the percentage of recovery.
Another crucial parameter is Precision , which measures the degree of agreement among individual test results when the procedure is applied repeatedly to multiple samplings of a homogeneous sample. Precision is usually expressed as the relative standard deviation (RSD) or coefficient of variation (CV) and is evaluated at different levels, including repeatability (intra-assay precision) and intermediate precision (inter-assay precision).
| Validation Parameter | Analyte: Diamorphine | Analyte: 6-Monoacetylmorphine | Analyte: Morphine |
|---|---|---|---|
| Quantification Range (ng/mL) | 1 – 1,000 | 1 – 1,000 | 1 – 1,000 |
| Intra-assay Accuracy (%) | 91 – 106 | 93 – 105 | 92 – 104 |
| Intra-assay Precision (CV %) | 2 – 9 | 3 – 8 | 3 – 7 |
| Extraction Recovery (%) | > 87 | > 90 | > 88 |
| Matrix Effect (%) | 99 – 125 | 101 – 118 | 100 – 115 |
This table provides an example of method validation data from a published LC-MS/MS method for the quantification of diamorphine and its metabolites in human plasma. Such data is essential to establish the reliability and performance of an analytical method.
Future Directions
- Developing Sensitive and Specific Analytical Methods: Further research is needed to develop more sensitive and specific analytical methods for the quantification of Pseudomorphine in complex matrices. [, , , , , ] This will enable accurate assessment of morphine degradation and facilitate its use as an analytical tool.
- Investigating its Biological Activity: While Pseudomorphine exhibits minimal morphine-like effects, its potential biological activities, particularly its cardiovascular effects, warrant further investigation. []
- Exploring its Role in Morphine Tolerance and Dependence: Although considered unlikely, investigating any potential role of Pseudomorphine in morphine tolerance and dependence could provide valuable insights into the complex pharmacology of morphine. []






Reviews
There are no reviews yet.