Buy Ethyl ether (Diethyl Ether) Cas 60-29-7
Buy Ethyl ether (Diethyl Ether) Cas 60-29-7
Ethyl Ether, Anhydrous, Reagent, ACS, is also referred to as sulfuric ether, diethyl ether or even just simply as ether. This liquid was historically used as an anesthetic, but in more recent years been replaced by other more effective anesthetics. It has however some other uses such as fuel and solvent. It’s an organic compound that is extremely flammable and volatile on top of being colorless. As an ACS grade Reagent, Spectrum Chemical manufactured compound is used as the quality standard against which other substances are grade and has met the toughest regulatory standards for quality and pureness.
Diethyl ether, or simply ether (abbreviated as eth.[8] or Et2O)[a][8] is an organic compound with the chemical formula (CH3CH2)2O, belonging to the ether class. It is a colourless, highly volatile, sweet-smelling (termed “ethereal odour”), extremely flammable liquid. It is a common solvent and was formerly used as a general anesthetic.[9]
Production
Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises:[10] Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%.[11]
- 2 CH3CH2OH → (CH3CH2)2O + H2O
Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.[12]
Uses
The dominant use of diethyl ether is as a solvent. One particular application is in the production of cellulose plastics such as cellulose acetate.[10]
Laboratory solvent
It is a common solvent for the Grignard reaction in addition to other reactions involving organometallic reagents.[13] These uses exploit its basicity. Diethyl ether is a popular non-polar solvent in liquid-liquid extraction. As an extractant, it is immiscible with and less dense than water.
Although immiscible, it has significant solubility in water (6.05 g/(100 ml) at 25 °C[2]) and dissolves 1.5 g/(100 g) (1.0 g/(100 ml)) water at 25 °C.[14]
Fuel
Diethyl ether has a high cetane number of 85–96 and, in combination with petroleum distillates for gasoline and diesel engines,[15] is used as a starting fluid because of its high volatility and low flash point. Ether starting fluid is sold and used in countries with cold climates, as it can help with cold starting an engine at sub-zero temperatures. For the same reason it is also used as a component of the fuel mixture for carbureted compression ignition model engines.
Ethyl ether
- Formula: C4H10O
- Molecular weight: 74.1216
- IUPAC Standard InChIKey: RTZKZFJDLAIYFH-UHFFFAOYSA-N
- CAS Registry Number: 60-29-7
- Chemical structure:
This structure is also available as a 2d Mol file or as a computed 3d SD file
View 3d structure (requires JavaScript / HTML 5) - Other names: Ethane, 1,1′-oxybis-; Anaesthetic ether; Anesthesia ether; Anesthetic ether; Diethyl ether; Diethyl oxide; Ethoxyethane; Pronarcol; Solvent ether; 1,1′-Oxybisethane; (C2H5)2O; Aether; Diaethylaether; Dwuetylowy eter; Etere etilico; Ether ethylique; Ether, ethyl; Ethyl ether, tech.; Ethyl oxide; Oxyde d’ethyle; Rcra waste number U117; UN 1155; 3-Oxapentane; Ether; Ethyl ether anhydrous A.C.S.; Sulfuric ether; NSC 100036
- Permanent link for this species. Use this link for bookmarking this species for future reference. Buy Ethyl ether (Diethyl Ether) Cas 60-29-7
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:
Data at NIST subscription sites:
- NIST / TRC Web Thermo Tables, “lite” edition (thermophysical and thermochemical data)
- NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data)
NIST subscription sites provide data under the NIST Standard Reference Data Program, but require an annual fee to access. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Gas phase thermochemistry data
Go To: Top, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry’s Law data, Gas phase ion energetics data, Ion clustering data, IR Spectrum, Mass spectrum (electron ionization), References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
ALS – Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein
GT – Glushko Thermocenter, Russian Academy of Sciences, Moscow
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔfH°gas | -252.7 ± 2.0 | kJ/mol | Ccb | Pihlaja and Heikkil, 1968 | Reanalyzed by Pedley, Naylor, et al., 1986, Original value = -250.3 ± 1.8 kJ/mol; ALS |
| ΔfH°gas | -252.2 ± 0.79 | kJ/mol | Cm | Pilcher, Skinner, et al., 1963 | ALS |
| ΔfH°gas | -244. | kJ/mol | Ccb | Murrin and Goldhagen, 1957 | ALS |
| Quantity | Value | Units | Method | Reference | Comment |
| ΔcH°gas | -2726.3 ± 1.8 | kJ/mol | Ccb | Pihlaja and Heikkil, 1968 | Corresponding ΔfHºgas = -276.9 kJ/mol (simple calculation by NIST; no Washburn corrections); ALS |
| ΔcH°gas | -2751.1 ± 0.75 | kJ/mol | Cm | Pilcher, Skinner, et al., 1963 | Corresponding ΔfHºgas = -252.1 kJ/mol (simple calculation by NIST; no Washburn corrections); ALS |
| Quantity | Value | Units | Method | Reference | Comment |
| S°gas | 342.2 | J/mol*K | N/A | Counsell J.F., 1971 | Other third-law entropy values at 298.15 K are 342.46 [ Cope C.S., 1959], 342.33 [ Stull D.R., 1969], and 342.60 J/mol*K [ Chao J., 1986].; GT |
Constant pressure heat capacity of gas
| Cp,gas (J/mol*K) | Temperature (K) | Reference | Comment |
|---|---|---|---|
| 62.50 | 100. | Chao J., 1986 | p=1 bar.; GT |
| 84.80 | 150. | ||
| 99.70 | 200. | ||
| 114.30 | 273.15 | ||
| 119.46 ± 0.15 | 298.15 | ||
| 119.86 | 300. | ||
| 142.81 | 400. | ||
| 165.77 | 500. | ||
| 186.35 | 600. | ||
| 204.35 | 700. | ||
| 220.04 | 800. | ||
| 233.74 | 900. | ||
| 245.68 | 1000. | ||
| 256.08 | 1100. | ||
| 265.12 | 1200. | ||
| 272.97 | 1300. | ||
| 279.81 | 1400. | ||
| 285.76 | 1500. |
Constant pressure heat capacity of gas
| Cp,gas (J/mol*K) | Temperature (K) | Reference | Comment |
|---|---|---|---|
| 121.94 | 309.98 | Counsell J.F., 1971 | Other experimental values of heat capacity [ Jennings W.H., 1934, Jatkar S.K.K., 1939, Valentin F.H.H., 1950] are believed to be less reliable (see [ Chao J., 1986]).; GT |
| 126.57 | 329.99 | ||
| 131.32 | 350.00 | ||
| 137.21 | 375.00 | ||
| 143.27 | 400.01 | ||
| 149.10 | 424.99 | ||
| 155.11 | 450.04 |
Condensed phase thermochemistry data
Go To: Top, Gas phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry’s Law data, Gas phase ion energetics data, Ion clustering data, IR Spectrum, Mass spectrum (electron ionization), References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
ALS – Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein
DH – Eugene S. Domalski and Elizabeth D. Hearing
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔfH°liquid | -271.2 ± 1.9 | kJ/mol | Ccb | Murrin and Goldhagen, 1957 | ALS |
| Quantity | Value | Units | Method | Reference | Comment |
| ΔcH°liquid | -2732.1 ± 1.9 | kJ/mol | Ccb | Murrin and Goldhagen, 1957 | Corresponding ΔfHºliquid = -271.1 kJ/mol (simple calculation by NIST; no Washburn corrections); ALS |
| Quantity | Value | Units | Method | Reference | Comment |
| S°liquid | 253.5 | J/mol*K | N/A | Counsell, Lee, et al., 1971 | DH |
| S°liquid | 252.7 | J/mol*K | N/A | Parks, Kelley, et al., 1929 | Extrapolation below 90 K, 58.6 J/mol*K. Revision of previous data.; DH |
| S°liquid | 283.3 | J/mol*K | N/A | Parks and Huffman, 1926 | Extrapolation below 90 K, 88.70 J/mol*K.; DH |
Constant pressure heat capacity of liquid
| Cp,liquid (J/mol*K) | Temperature (K) | Reference | Comment |
|---|---|---|---|
| 172.5 | 298.15 | Counsell, Lee, et al., 1971 | T = 15 to 300 K.; DH |
| 171.88 | 293.15 | Mazur, 1939 | T = -112 to 20°C.; DH |
| 172.0 | 293. | Mazur, 1939, 2 | T = -110 to 20°C.; DH |
| 167.4 | 290. | Kurnakov and Voskresenskaya, 1936 | DH |
| 164.8 | 255.2 | Aoyama and Kanda, 1935 | T = 80 to 255 K. Value is unsmoothed experimental datum.; DH |
| 179.9 | 308. | Bennewitz and Wendroth, 1927 | T = 308 to 488 K. Value is unsmoothed experimental datum. Pressure 40 atmospheres.; DH |
| 170.7 | 290.0 | Parks and Huffman, 1926 | T = 76 to 290 K. Value is unsmoothed experimental datum.; DH |
| 179.1 | 286.6 | Keyes and Beattie, 1924 | T = 274, 286 K.; DH |
Phase change data
Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Henry’s Law data, Gas phase ion energetics data, Ion clustering data, IR Spectrum, Mass spectrum (electron ionization), References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
BS – Robert L. Brown and Stephen E. Stein
TRC – Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director
ALS – Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein
AC – William E. Acree, Jr., James S. Chickos
DH – Eugene S. Domalski and Elizabeth D. Hearing
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| Tboil | 307.7 ± 0.4 | K | AVG | N/A | Average of 20 out of 21 values; Individual data points |
| Quantity | Value | Units | Method | Reference | Comment |
| Tfus | 154. ± 7. | K | AVG | N/A | Average of 13 out of 14 values; Individual data points |
| Quantity | Value | Units | Method | Reference | Comment |
| Ttriple | 156.92 | K | N/A | Wilhoit, Chao, et al., 1985 | Uncertainty assigned by TRC = 0.05 K; TRC |
| Ttriple | 149.86 | K | N/A | Counsell, Lee, et al., 1971, 2 | Crystal phase 2 phase; Uncertainty assigned by TRC = 0.02 K; TRC |
| Ttriple | 156.92 | K | N/A | Counsell, Lee, et al., 1971, 2 | Crystal phase 1 phase; Uncertainty assigned by TRC = 0.02 K; TRC |
| Ttriple | 156.8 | K | N/A | Parks and Huffman, 1926, 2 | Uncertainty assigned by TRC = 0.2 K; TRC |
| Quantity | Value | Units | Method | Reference | Comment |
| Tc | 467. ± 2. | K | AVG | N/A | Average of 29 out of 30 values; Individual data points |
| Quantity | Value | Units | Method | Reference | Comment |
| Pc | 36. ± 1. | bar | AVG | N/A | Average of 16 out of 17 values; Individual data points |
| Quantity | Value | Units | Method | Reference | Comment |
| Vc | 0.274 | l/mol | N/A | Kobe, Ravicz, et al., 1956 | Uncertainty assigned by TRC = 0.005 l/mol; TRC |
| Quantity | Value | Units | Method | Reference | Comment |
| ρc | 3.5 ± 0.4 | mol/l | AVG | N/A | Average of 10 values; Individual data points |
| Quantity | Value | Units | Method | Reference | Comment |
| ΔvapH° | 27.1 ± 0.5 | kJ/mol | AVG | N/A | Average of 6 values; Individual data points |
Diethyl ether
[CAS# 60-29-7]
Diethyl Ether vs Ethyl AcetateDiethyl Ether, with the chemical formula C4H10O and CAS number 60-29-7, is a colorless liquid characterized by its high volatility and flammability. Its structure consists of two ethyl groups attached to a single oxygen atom, classifying it as an ether. Commonly known for its rum-like odor, diethyl ether is widely used as a solvent, a general anesthetic, and in laboratory procedures. Despite its versatility, it is sensitive to light and air, often forming explosive peroxides upon exposure. Ethyl Acetate, with the chemical formula C4H8O2 and CAS number 141-78-6, is a carboxylate ester formed from ethanol and acetic acid. This colorless liquid, with a fruity odor, serves as a widely used solvent in various industries. Its melting point is -83.6°C, and it boils at 77°C. Ethyl acetate is known for its low toxicity and cost-effectiveness, making it a preferred choice for applications in adhesives, paints, and flavorings. Its hydrolysis in the presence of a strong base yields ethanol and acetate. Diethyl ether and ethyl acetate are both essential organic solvents with unique characteristics. While both share applications in industrial and laboratory settings, their chemical structures and physical properties dictate their specific uses and limitations. Structural Analysis of Diethyl Ether vs Ethyl AcetateMolecular Composition and Properties

 Synthesis Methods
Both compounds are synthesized using ethanol but differ significantly in their reaction pathways and catalysts. Diethyl ether production focuses on ether bond formation, whereas ethyl acetate synthesis emphasizes esterification. Applications: Diethyl Ether vs Ethyl AcetateDiethyl ether and ethyl acetate are indispensable solvents in industrial and laboratory settings, each possessing unique properties that define their specific uses. While diethyl ether shines in chemical synthesis and laboratory applications, ethyl acetate is a cornerstone of diverse industrial processes, including manufacturing and consumer goods.
Diethyl Ether in Laboratory and Medical UseDiethyl ether is a cornerstone solvent in organic chemistry laboratories, prized for its remarkable ability to dissolve nonpolar substances. This characteristic makes it indispensable in various synthesis and extraction processes, particularly in reactions involving Grignard reagents. The low boiling point of diethyl ether enhances its utility, allowing for easy recovery of the solvent after the reaction, thereby improving efficiency in laboratory workflows. Additionally, it serves as an effective medium for recrystallization and separation processes, ensuring high-purity outcomes in chemical research and development. Historically, diethyl ether played a significant role in the medical field as one of the first general anesthetics. Its rapid onset and effectiveness in inducing unconsciousness revolutionized surgical procedures during the 19th and early 20th centuries. However, due to its flammability and the availability of safer anesthetics, its medical use has significantly declined. Today, its primary applications remain in the laboratory, where its chemical properties are leveraged for research and experimental purposes, particularly in academic and industrial chemistry settings. Ethyl Acetate in Industrial ApplicationsEthyl acetate is a versatile solvent with extensive applications across various industries. Its low toxicity, mild odor, and effective solvency make it a preferred choice in the production of adhesives, paints, and coatings. In the printing industry, ethyl acetate is widely used in the formulation of inks for flexographic and gravure printing processes due to its ability to provide fast evaporation and superior adhesion on diverse surfaces. These properties ensure high-quality results in packaging and labeling applications. Beyond its industrial use as a solvent, ethyl acetate plays a vital role in consumer goods. In the food and beverage industry, it is employed as a flavoring agent, adding fruity and sweet notes to candies, baked goods, and beverages. Additionally, ethyl acetate is integral to the decaffeination process for coffee and tea, providing a solvent option that meets strict food safety standards. The cosmetic industry also benefits from its inclusion in nail polish removers, where its effectiveness in dissolving lacquer is combined with a relatively pleasant odor compared to alternative solvents. These wide-ranging applications highlight ethyl acetate’s importance as a solvent that balances functionality with consumer safety and usability. Disadvantages of Diethyl Ether vs Ethyl Acetate
|
||||||||||||||||||
| CAS: 60-29-7 Product: Diethyl ether No suppilers available. |
||||||||||||||||||
| Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7, Buy Ethyl ether (Diethyl Ether) Cas 60-29-7 |
| Classification | Organic raw materials >> Ether compounds and their derivatives >> Ether, ether alcohol |
|---|---|
| Name | Diethyl ether |
| Synonyms | Ethyl ether |
| Molecular Structure | 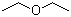 |
| Molecular Formula | C4H10O |
| Molecular Weight | 74.12 |
| CAS Registry Number | 60-29-7 |
| EC Number | 200-467-2 |
| SMILES | CCOCC |
| Density | 0.7±0.1 g/cm3 Calc.*, 0.706 g/mL (Expl.) |
|---|---|
| Melting point | -116 ºC (Expl.) |
| Boiling point | 33.2±3.0 ºC 760 mmHg (Calc.)*, 34.6 ºC (Expl.) |
| Flash point | -40.0 ºC (Calc.)*, -45 ºC (Expl.) |
| Solubility | water: 69 g/L (20 ºC) (Expl.) |
| Index of refraction | 1.361 (Calc.)*, 1.353 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |   GHS02;GHS07 DangerGHS02 Details GHS02;GHS07 DangerGHS02 Details |
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H224-H302-H336 Details | ||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P261-P264-P270-P271-P280-P301+P317-P303+P361+P353-P304+P340-P319-P330-P370+P378-P403+P233-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 1155 | ||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||
| Diethyl ether, with the chemical formula C2H5OC2H5, is a simple aliphatic ether consisting of two ethyl groups linked by an oxygen atom. It is a volatile, highly flammable liquid with a characteristic sweet odor and low boiling point, which makes it one of the earliest and most widely used organic solvents.
The discovery of diethyl ether dates back to the 16th century when it was first prepared by German physician and chemist Valerius Cordus in 1540 through the acid-catalyzed dehydration of ethanol. Its anesthetic properties were recognized in the 19th century, with William T. G. Morton famously demonstrating its use as a surgical anesthetic in 1846. This landmark event established diethyl ether as the first widely used general anesthetic, revolutionizing surgery by enabling pain-free operations. Diethyl ether’s primary applications are as a solvent and an anesthetic. In chemical laboratories and industry, it is valued for its ability to dissolve a wide range of organic compounds due to its moderate polarity and low reactivity. It is commonly used in extractions, recrystallizations, and as a reaction medium for Grignard reagents and other organometallic reactions. Its low boiling point facilitates easy removal by evaporation after completion of a reaction or purification process. Historically, the anesthetic use of diethyl ether had a profound impact on medicine. It allowed surgeons to perform longer and more precise operations without causing extreme pain, significantly reducing surgical mortality and expanding the possibilities of surgical procedures. Although largely replaced by modern anesthetics due to flammability and other side effects, ether remains an important historical milestone in medical practice. Industrial production of diethyl ether involves the acid-catalyzed dehydration of ethanol using sulfuric acid under controlled conditions. The reaction produces ether alongside water, and the product is purified by distillation. Safety is a major consideration because diethyl ether forms highly flammable peroxides upon exposure to air and light, which can be explosive if concentrated. Proper storage and handling are therefore critical. In modern use, diethyl ether continues to serve as a versatile solvent in laboratories, particularly for organic synthesis and purification processes. Its role in the development of anesthesia also secures its place as a historically significant chemical, illustrating the intersection of organic chemistry and medicine. |
Detta Thermo Scientific Chemicals varumärkesprodukt var ursprungligen en del av Acros Organics produktportfölj. Viss dokumentation och etikettinformation kan referera till det äldre varumärket. Den ursprungliga Acros Organics-produkt-/artikelkoden eller SKU-referensen har inte ändrats som en del av varumärkesövergången till Thermo Scientific Chemicals .
SpecifikationerEmpty heading
Specifikationer
| Kemiskt namn eller material | Diethyl ether |
| Smältpunkt | -116.0°C |
| Densitet | 0.7140g/mL, 0.714 |
| Kokpunkt | 34.6°C |
| Kvantitet | 25 L |
| Flampunkt | −45°C |
| Infrarött spektrum | Authentic |
| Förpackning | Metalltrumma |
| Fysisk form | Vätska |
| Brytningsindex | 1.3510 to 1.3530 |
| Visa mer |
-
Chemical Formula: C₂H₅₂O
-
Molecular Weight: 74.12 g/mol
-
-
Diethyl ether | 60-29-7 – ChemicalBook
Jan 13, 2026 · Diethyl ether (CAS 60-29-7) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight, uses, prices, suppliers, SDS …
-
Boiling point: 34.6 °C (lit.)
-
Melting point: 116 °C
-
Density: 0.714
-
vapor density: 2.6 (vs air)
-
Diethylene Glycol Monoethyl Ether
Diethylene Glycol Monoethyl Ether (CAS 111-90-0) information, including chemical properties, structure, melting point, boiling point, density, formul…
-
-
Diethyl ether for spectroscopy Uvasol 60-29-7 – MilliporeSigma
≥98% (GC), contains 2% ethanol as stabilizer, suitable for UV/Vis spectroscopy, Uvasol® CAS Number: 60-29-7; Synonyms: Ethyl ether, Ether, Et2O, Ethoxyethane,Ether,Ethyl ether; Linear …
-
Diethyl ether CAS 60-29-7 | 100921
Synonyms: Et2O, Ethoxyethane, Ethyl ether, Ether CAS #: 60-29-7 EC …
-
CAS number: 60-29-7
-
Description: Diethyl ether
-
Catalogue Number: 100921
-
Synonyms: Et2O, Ethoxyethane, Ethyl ether, Ether
-
-
CAS RN 60-29-7 | Fisher Scientific
CAS: 60-29-7 Molecular Formula: C4H10O Molecular Weight (g/mol): 74.12 MDL Number: MFCD00011646 InChI Key: RTZKZFJDLAIYFH-UHFFFAOYSA-N IUPAC Name: …
-
-
QUALIKEMS Diethyl Ether 500ml CAS NO-60-29-7 (Lab use only) : Amazon.in …CAS No : 60-29-7 | Product Name : Diethyl Ether | PharmaffiliatesDiethyl ether CAS 60-29-7 | 100921【永山化工】乙醚 日本試藥 大量現貨 Diethyl ether 60-29-7 500ml | 蝦皮購物Diethyl Ether Anhydrous ACS Grade | CH5027 | CAS 60-29-7 | ChemieRISOLAB DIETHYL ETHER – CAS No: 60-29-7 – ISOLAB Laborgeräte GmbHEthyl ether (CAS 60-29-7) – Chemical & Physical Properties by CheméoDiethyl Ether – Clear, Colorless Liquid | Cas: 60-29-7, Molecular …Diethyl ether (CAS 60-29-7) | Glentham Life SciencesDiethyl ether CAS 60-29-7 | 100923Eter dietylowy – czysty / Diethyl ether / CAS 60-29-7CAS-60-29-7, Diethyl Ether Dry AR (Solvent Ether, Ethyl Ether) Meets …C4H10O diethyl ether CAS 60-29-7 chemical substance in white plastic …Ether diéthylique CAS 60-29-7 | 100930CAS No. 60-29-7 – Diethyl Ether – AccuStandardDiethyl ether | CAS#:60-29-7 | ChemsrcDiethyl Ether | CAS No- 60-29-7 | Simson Pharma Limited
-
-
60-29-7 CAS | DIETHYL ETHER | High Purity …
DIETHYL ETHER, 60-29-7, High Purity Solvents, (C2H5)2O by Loba Chemie, India
-
Diethyl Ether 60-29-7 | TCI AMERICA
Diethyl Ether | (C2H5)2O or C4H10O | CID 3283 – structure, chemical names, physical and chemical properties, classification, patents, literature, …
-
CAS # 60-29-7, Diethyl ether, Ethyl ether – chemBlink
chemBlink provides information about CAS # 60-29-7, Diethyl ether, Ethyl ether. An open source of chemical information available to the public online since 2005.
-
Diethyl ether, 1 l, glass, CAS No. 60-29-7 | A to Z – Carl Roth
Examples of effect: Flammable; liquids form compounds with the air which can become explosive; produce flammable gases with water or can self-combust. Safety: Keep away from open …
-
Diethyl Ether MATERIAL SAFETY DATASHEET CAS No 60-29-7 …
Diethyl ether CAS-No. EC-No. Index-No. 60-29-7 200-467-2 603-022-00-4 Flam. Liq. 1; Acute Tox. 4;
-
Diethyl ether 60-29-7 – sigmaaldrich.cn
Firstly, PAE made of 1,4-butanediol diacrylate and 4,4′-trimethylene dipiperidine is used as a pH-sensitive Oxidation of secondary alcohols in diethyl ether with aqueous chromic acid








Reviews
There are no reviews yet.